HELLO! MY NAME IS
Omid Bafkar
Mechanical Design Engineer
I am a passionate and driven product development engineer dedicated to guiding innovations from competitive analysis through validation. Leveraging FEA/CFD, DFX, and rapid prototyping, I ensure exceptional technical performance and compliance with ISO 13485 standards. With a focus on creating market-ready solutions that deliver standout user experiences and lasting satisfaction, I strive to transform complex requirements into impactful, user-centered products.
Success Rate in Design-to-Production Transfer
Saved by Accelerating Iterative User Testing Cycles
Reduction in Assembly Steps via DFMA Integration
About Me
I am a Senior Mechanical Engineer with over a decade of experience driving end-to-end product innovation in complex medical and consumer health markets. My mission is to translate high-stakes technical requirements—such as those found in biosensors and microfluidics—into products that are not only clinically effective but also intuitive and reliable for the end-user. I achieve this by relentlessly bridging the gap between advanced FEA/CFD analysis and essential user factors like ergonomics, usability, and sensory analysis. My unique value lies in my cross-functional leadership; I collaborate seamlessly with R&D, clinical, and manufacturing teams to ensure that every concept is aligned with ISO 13485 compliance and optimized for manufacturability (DFM/DFMA), resulting in products that are both successful in the market and enduringly satisfying for the people who use them.

Services

Microfluidic & Complex Mechanism Design
Full-cycle design of electromechanical, thermal, and microfluidic systems. Expertise in 3D CAD (SolidWorks/Fusion 360), Stepper Motor integration, and translating theoretical concepts into high-reliability hardware.
CFD/FEA & Risk-Based Performance Analysis
Applying advanced CFD/FEA to optimize thermal and stress performance. Conducting V&V, FMEA, and Root Cause Analysis to ensure risk-based design compliance and repeatable, real-world product reliability.
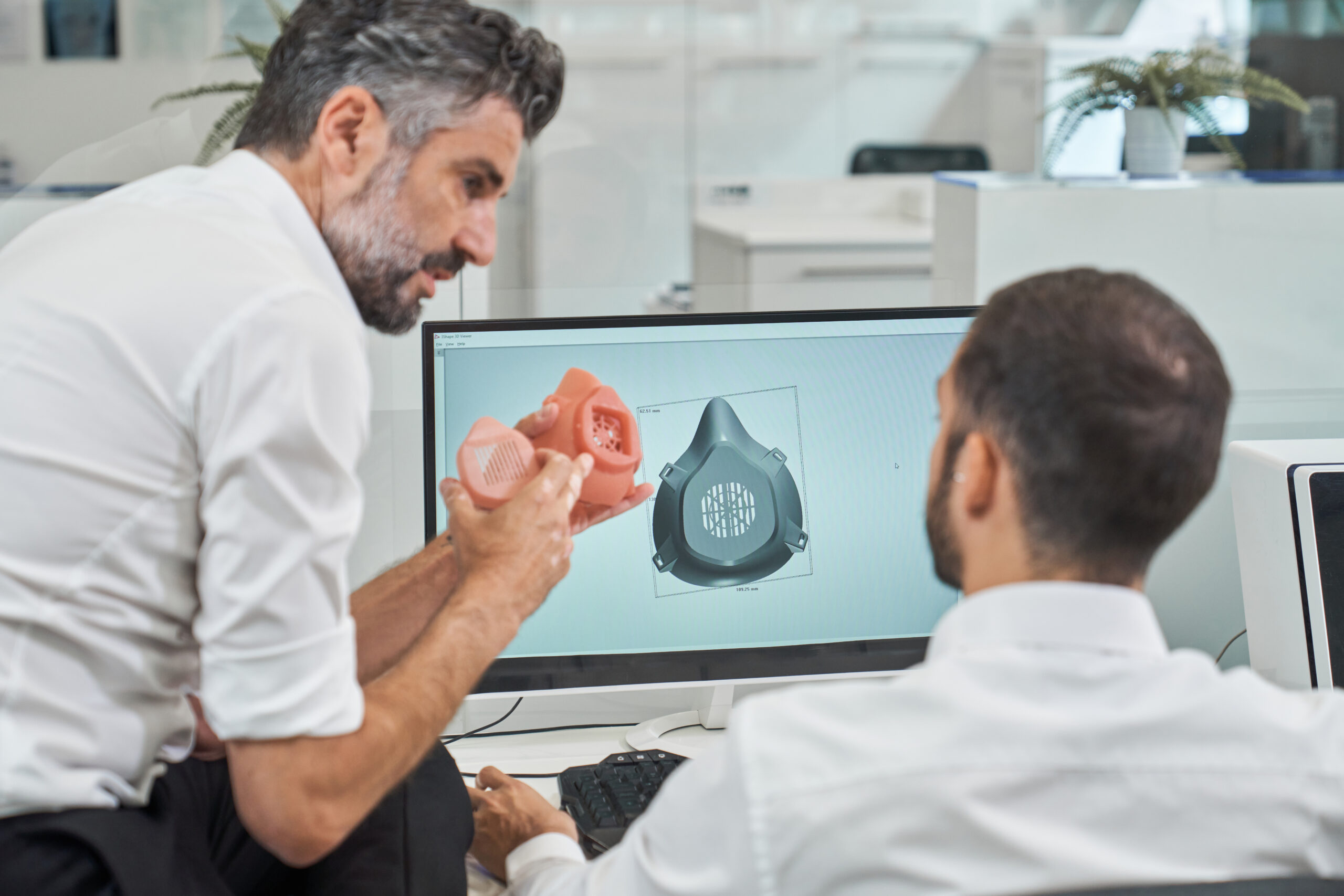
User-Centric DFX & Production Transfer
Driving innovation from UI/UX workflows and sensory testing through DFM/DFMA. Expertise in working with international molding teams and ensuring seamless, compliant ISO 13485 design-to-production transfer.
Language Skills
English
French
Persian
Hard Skills
Ready to bring your ideas to life? I'm here to help
I’m always open to discussing new opportunities in Biosensor, Microfluidic, or Medical Device R&D where a blend of deep mechanical engineering and user-centric design is critical. If you are seeking senior leadership for complex product development challenges, cross-functional project oversight, or expertise in achieving ISO 13485 compliant manufacturing transfer, please feel free to reach out. Let’s discuss how my 10+ years of end-to-end innovation can translate your technical vision into a successful, market-ready product.